Clean water is an essential part of our daily lives and, thankfully, more people now have access to it than ever before. The water we drink and use is sorted into two broad categories: hard and soft.
Soft water has low mineral content, whereas hard water has high mineral content and contains calcium and, to a lesser extent, magnesium (1). Hard water won’t negatively affect your health, but it can damage your home’s plumbing and outlets such as faucets and showers.
Fortunately, it is now very easy to reduce the hardness of your water. In this article, we will explain how to remove calcium from your water using four different methods. We will also explore why you should consider doing so.
Key Takeaways
- Soft water has low mineral content, while hard water has high mineral content, including calcium and magnesium.
- Hard water can damage home plumbing and appliances, but it’s not harmful to health.
- Methods to remove calcium from water include using water filters, chemical treatment, water softeners, and boiling water.
- Hard water can have some benefits, such as providing calcium for teeth and bones, but softening water may be necessary in certain situations.
What Makes Water Soft?
When water is in its natural forms — such as in the ocean or raindrops — it is soft. As it flows through or over the ground, rocks, and mineral-rich environments, it collects calcium and magnesium.
Hardness is measured by the percentage of these minerals, in this case, calcium. Water with less than 17 milligrams of calcium per liter is considered soft.
If the water contains between 17 and 60 milligrams of calcium per liter, it’s slightly hard. Between 60 and 120 milligrams per liter is moderately hard, and 120 milligrams or higher per liter is considered very hard (2).
How to Tell Hard Water from Soft
The quickest way to determine if your water is hard is to drink it. You can taste the minerals in hard water, just as you can taste the sodium in soft water. When we remove the minerals from water, people often describe its taste as flat and slightly salty.
Hard water leaves a residue. When it evaporates, calcium and other minerals will be left behind as solid deposits — this is what causes limescale in your home.
Hard water can also affect your skincare and grooming products. They won’t lather as much, so they might be less effective than they are in soft water. Hard water can also leave a white residue on your skin.
How is Hard Water Softened?
There are several ways to remove calcium from water, but most methods are used for industrial or commercial purposes.
Ion-Exchange Resin
This is achieved by attaching a water softening device directly to your supply. The containers hold ion-exchange resin or beads that exchange hard molecules for soft ones. During this process, calcium and magnesium are replaced with sodium and occasionally potassium.
Calcium Hydroxide
Calcium hydroxide is a powder used to treat and prepare water, food, and paper. It purifies water and is used to raise the alkalinity of freshwater to prevent rust.
This method is typically restricted to industrial use, as handling calcium hydroxide can be dangerous.
Distillation
Distillation is an effective but expensive method to reduce calcium in your water. As water is heated, it evaporates and leaves behind hard water ions. The main issue is that the energy required to continually provide soft water is not cost-effective in a residential setting.
Chelating Agents
Chelators — molecules that easily bind to metal molecules — can remove calcium and magnesium from water. These substances are commercially added to cleaning products, including shampoos, soaps, and detergents.
Although they are prominent in their respective industries, they do have an environmental impact, which is why they are losing favor as water softeners.
Reverse Osmosis
Reverse osmosis utilizes controlled pressure to isolate hard water ions. The purified water is then pushed through a filter to remove calcium and magnesium.
One benefit of this method is that it doesn’t replace hard water ions with other molecules. However, reverse osmosis machines require regular maintenance to keep them effective.
Know The Difference
How to Remove Calcium From Water
There are many possible reasons to soften your water supply. Whether you do it for skin care and grooming, to protect your clothes, or to prolong the life of your plumbing appliances, there are multiple methods you can try at home.
Here are four quick and easy ways to remove calcium from your water supply:
Use a Water Filter
The easiest and most convenient way to soften your water is to use a water filter. Most filters use activated carbon to absorb and trap contaminants. They can filter a variety of impurities, from chlorine to heavy metals.
You will need to spend some money on a calcium water filter, and they require installation. They not only soften your water supply, but they also clean it. This is an example of reverse osmosis.
Pros
- Effortless to use
- Water is purified and safer to drink
- Easy to find
Cons
- Filters need replacing
- Smaller filters won’t protect your plumbing
Chemical Treatment
You don’t need a chemistry degree to treat your water. All you need is some sodium carbonate or washing soda. Adding this to hard water will break down calcium and magnesium and remove them from your water supply.
Chemical treatment can protect your pipes and is safe when used as intended. Unfortunately, this involves using a cleaning agent which means it is unsafe for consumption (3).
There are other chemicals that you can use to clean, purify or soften water. These aren’t easy to acquire or use and are typically reserved for water treatment plants (4).
Pros
- Best for maintenance
- Removes all traces of calcium
Cons
- Unsafe for drinking
- Difficult to acquire and use
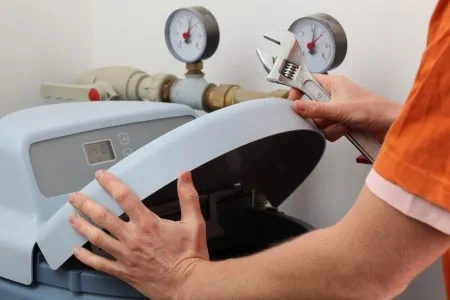
Water Softeners
A water softener provides the same effects as chemical treatment, but without adding harmful substances to your supply. It is a device that replaces calcium and magnesium with sodium to alter the structure of your water.
Once this exchange takes place, the softener will filter out calcium and other impurities. This leaves you with cleaner soft water, with no effort on your part. These machines are the most popular choice for ion exchange.
Pros
- Safe to drink
- Cleans your water supply
- Automated
Cons
- Installation required
- Expensive
Boil Your Water
Heat will help you remove calcium from water naturally. Traces of calcium and other impurities will settle at the bottom of the container and can be easily removed with a strainer. This method might be the most basic, but it isn’t effective on a large scale.
The upside is that no chemicals or special devices are necessary, and it leaves your water safe for any use including drinking. This process is distillation, so keep in mind that while it is highly effective, it is also time-consuming.
Pros
- Easy to do
- No special tools are needed
- Safe to use and drink
Cons
- Uses more energy
- Only useful for small volumes of water
Should You Soften Your Water?
Hard water reduces your salt intake and is a useful source of calcium if you aren’t receiving much from your diet. Research suggests that it could have a positive effect on cardiovascular health (5). Calcium in hard water also benefits your teeth and bones, especially for children.
Softening your water is recommended in some situations but in others, hard water can be better. One example of this is a swimming pool. Although soft water feels better on your skin, it can damage the integrity of your pool’s walls (6).
Take the time to test your water and consider your individual plumbing setup, as there is no universal answer regarding whether hard or soft water is better.